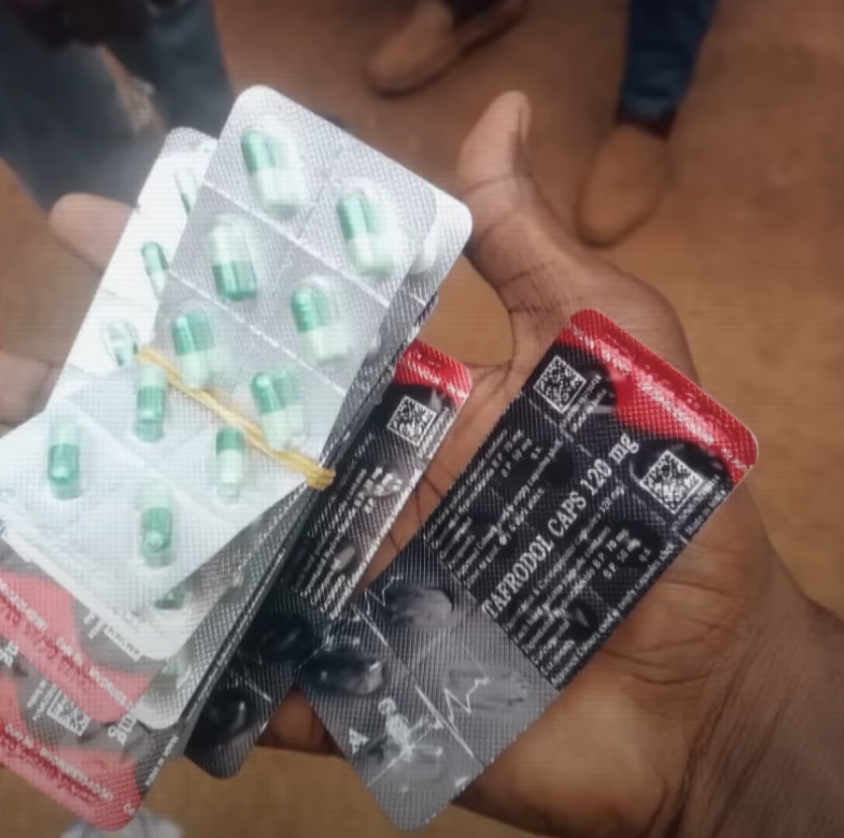
India has banned the production and export of two addictive drugs, tapentadol and carisoprodol, after an investigation linked them to a growing opioid crisis in West Africa.
The move comes after a BBC report exposed that a Mumbai-based company, Aveo Pharmaceuticals, was illegally shipping these drugs to countries like Nigeria, Ghana, and Ivory Coast.
Secretly recorded footage showed an Aveo director admitting that the drugs were harmful but profitable.
Following this exposure, Indian authorities raided the company’s factory and seized large amounts of the drugs to stop further distribution.
The Indian health ministry announced that it had revoked manufacturing and export licenses for the combination, calling it a serious health risk. Authorities also shut down all operations of Aveo Pharmaceuticals after an audit by India’s drug regulatory bodies.
Tapentadol is a powerful opioid, while carisoprodol is a muscle relaxant that can be addictive. When combined, they pose serious health risks, including breathing problems, seizures, and fatal overdoses.
Although this combination is banned in India for medical use, illegal exports have continued, fueling drug abuse in West Africa.
Reports estimate that about four million people in Nigeria misuse opioids. The World Health Organization (WHO) has also warned that 100,000 people in Africa die every year due to fake or poor-quality medicines.
The BBC investigation found that Aveo Pharmaceuticals and its sister company, Westfin International, had sent millions of these pills to West Africa, where they were sold openly on the streets.
In a secretly recorded conversation, Aveo director Vinod Sharma admitted that the pills were dangerous but still a profitable business. He also confirmed that taking multiple pills could make users feel high.
In response, Aveo Pharmaceuticals denied all accusations, saying they always followed regulations. However, Indian drug regulators clarified that while both drugs are approved individually, their combination is illegal.
Officials also revealed that other companies were making similar illegal drugs, and legal action was being taken against them.
India’s Food and Drugs Administration has promised stricter monitoring to prevent illegal drug exports in the future.
Dr. Rajeev Singh Raghuvanshi, the head of India’s drug control department, confirmed that manufacturing and export licenses for the combination had been revoked due to the drugs’ high abuse potential.
This crackdown aims to protect public health and prevent India’s pharmaceutical industry from being misused for illegal drug trade.